Thursday, September 4, 2008
AREEEE YOU READYYYYYYYY KIDS?!
Physics is good(y) I like physics.
I can even READ physics with a swollen eye :B
Firstly we're going to touch on WHAT IS INTERNAL ENERGY!
WHAT IS INTERNAL ENERGY.
Internal energy is the amount of energy needed to raise the temperature.
Ok, that's number one, easy? Easy peasy 8)
(btw my eye is still like a fishball, i hate my eye yuck)
Secondly, Melting and Solidification!
Melting: From a solid state to a liquid state.
Solidification: from a liquid state to a solid state.
OKAY THAT WAS EASY! RIGHT?!?!?!?!?!?!?! 8)
Next, we've.. Boiling and Condensation.

Boiling: liquid changing to vapour.
It occurs at a fixed temperature, quick in process, takes place throughout the liquid,
bubbles are formed, temperature remains constant, thermal energy supplied by energy source.

Condensation: It is the reverse of boiling 8)
Lastly, we've Evaporation!
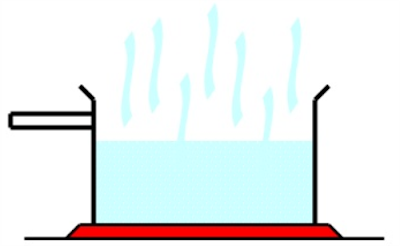
It is similiar to boiling, it is a process where liquid is changed into gaseous state.
However it's different as it has different conditions than boiling.
1. Occurs at ANY temperature.
2. Slowwwwwwwwwwwwwww.
3. Takes place only at the liquid surface.
4. No bubble formed.
5. Temperature varies.
6. Thermal energy supplied by surroundings.
That's all! ;) See! Physics is not as bad as you think!
Now... videos! Watch 2 boost your physics yum yum intelligence(y) !
I'm back to teach you all chapter 8.
Ok my eye is sore and I look like I just got punched :/
Omg I even feel like I got punched.
Okay, chapter 8 now chapter 8 yay yay yay! 8) So exciting.
no la haha i know it's not exciting i know i know~
CONDUCTION.
Convection is the transfer of heat by the actual movement of the warmed matter. Heat leaves the coffee cup as the currents of steam and air rise. Convection is the transfer of heat energy in a gas or liquid by movement of currents. (It can also happen is some solids, like sand.) The heat moves with the fluid. Consider this: convection is responsible for making macaroni rise and fall in a pot of heated water. The warmer portions of the water are less dense and therefore, they rise. Meanwhile, the cooler portions of the water fall because they are denser.
CONVECTION

Conduction is the transfer of energy through matter from particle to particle. It is the transfer and distribution of heat energy from atom to atom within a substance. For example, a spoon in a cup of hot soup becomes warmer because the heat from the soup is conducted along the spoon. Conduction is most effective in solids-but it can happen in fluids.
(Oh my god Im exhausted already, my eye..)
Now, factors that actually AFFECT the rate of radiation!
WHEN GOOD CONDUCTORS OF HEAT LIKE:
Cooking Utensils, Iron rods and heat exchangers!
ARE USED,
the energy then is transferred quickly through a substance.
WHEN BAD CONDUCTORS OF HEAT LIKE:
Table mats, Sawdust, Wooden Ladles, Woollen clothes!
ARE USED...
it would insulate the heat and minimise loss of thermal energy
and prevents thermal energy from being transferred quickly!
That's it for chapter 8, do re read and re read till you get it.
OHHHHHHHHHHHHHH MY GOD BLESS MY EYE.
OHHH YOUTUBE IS GODDDDDDDDDDDDDDDDDDDDDDDDDDD! 8)
Everyone listen, okay no read carefully.
Aim is to absorb something into thee very important brain ;)
THE STATES OF MATTER.
Solid water- frozen water and less dense than water. Example: Ice
Liquid water- is wet and fluid. For example: A glass of water!
Water as a gas—vapor is always present in the air around us, not able to be seen.
CLEAR?
Yes - thank you, you can scroll down. So smart!
No - Repeat reading again! Not stupid but just abit slow! ;)
Above are just definations of states of matters.
Now, we're going on to their properties!!!

(Heart shaped ice cubes!)
Solids have fixed shape and volume. (you can't possible put 1000 icecubes in a cup!)
Hard and rigid; large force needed to change its shape. (you can't change a square icecube to become a circle, can you?)
High density and incompressible. (COMPRESS ICE?!!?!?!?!?!?!? :/
Liquids have a fixed volue but no fixed shape.
It has a hgih density and it is incompressible 8)
Lastly, gases have no fixed shape neither fixed volume.
it has a low density and it is compressible!
EASY? YES? NO? Btw Im eating Ritter Sport now hehehe (y)
Now we've reached the kinetic model of matter!
Molecules in Gaseous state is packed very far apart while in the Solid state is packed very close together.
Remember: Solid first liquid second third gas ;)
And also, the closer the particles are packed together, the higher density they have
Now, for chapter 7, we're only left with BROWNIAN MOTION.
ok.. doesn't sound very appealing but indeed it's pretty interesting.
Okay whatever I can't seem to change my font back so I'm not going to change it back.
SO.. WHAT IS THE BROWNIAN MOTION?
To explain it in the very very super duper complicated way, it is:
Brownian motion (named in honor of the botanist Robert Brown) is the random movement of particles suspended in a liquid or gas or the mathematical model used to describe such random movements, often called a particle theory.
In a simple and very comprehendable way, it is:
- A MOTION OF SMOKE PARTICLES.
- HIGHER TEMPERATURE, SMOKE PARTICLES MOVE MORE VIGOROUSLY.
Okay please tell me you could actually understand that.
UH HUH! Einstein's Explanation of Brownian Motion!
http://galileoandeinstein.physics.virginia.edu/more_stuff/Applets/brownian/brownian.html
Yes very important do take a look because it affects your understanding about this motion.
;)
Till then, thank you goodbye and God bless you Miss Wan!



